Micro Systems Blog // Why ISO Class 7 Cleanrooms Are Essential for Next-Gen Medical Device Moulding
|
Getting your Trinity Audio player ready...
|
In the precision-driven field of medical device manufacturing, the integration of cleanroom injection moulding is no longer a competitive advantage but a baseline requirement for quality, safety, and compliance. As regulatory scrutiny intensifies and devices become increasingly complex, only the most advanced manufacturers can meet the technical and operational demands of cleanroom-capable production environments. In this blog, Micro Systems explores why ISO Class 7 cleanroom injection moulding has become a vital standard for producing precision medical components, and how Micro Systems is leading the way with its new facility.
What Is Cleanroom Injection Moulding?
Cleanroom injection moulding is the process of producing plastic components within a controlled environment where contamination by airborne particulates, microbes, and chemical vapours is minimised. This process ensures that critical components used in healthcare, ranging from surgical instruments to drug-delivery devices, meet the highest sterility and biocompatibility standards.
Cleanrooms are classified under ISO 14644-1, with ISO Class 7 being one of the most commonly used cleanroom environments for medical moulding. More sensitive applications, such as implantables or diagnostics, may require ISO Class 5 or 6.
Requirements for ISO Class 7 Cleanroom Certification
1. Airborne Particulate Count
- Maximum allowable particles per cubic meter:
– ≥0.5 µm: 352,000 particles/m³
– ≥5.0 µm: 2,930 particles/m³
- Measured using calibrated particle counters across multiple sampling points.
2. Air Changes Per Hour (ACH)
- 60–90 air changes per hour are typical.
- Achieved through HEPA-filtered HVAC systems to continuously scrub air of particulates.
- The design ensures a consistent flow of clean air while maintaining positive pressure. HEPA Filtration
- High-Efficiency Particulate Air (HEPA) filters rated at 99.97% efficiency for particles ≥0.3 µm.
- Supplemented with ULPA filters (99.999% efficiency) depending on application.
4. Pressure Differentials
- Cleanrooms must maintain positive air pressure relative to adjacent lower-classified or uncontrolled areas.
- A differential pressure of ≥15 Pascals is commonly maintained.
5. Temperature & Humidity Control
- Typical range: 18°C to 22°C and 30%–50% relative humidity, depending on material and process sensitivity.
- These conditions prevent electrostatic discharge (ESD), material degradation, and microbial growth.
6. Surfaces and Construction Materials
- Non-porous, smooth, and easy-to-clean surfaces.
- Materials must not shed particles or off-gas under operational conditions.
- Coved corners, flush-mounted lighting fixtures are common to reduce particle traps.
7. Personnel and Gowning Protocols
- Personnel must wear full cleanroom garments: hairnets, gloves, coveralls, masks, and shoe covers.
- Strict protocols for entry/exit, hand hygiene, and movement minimise contamination.
8. Environmental Monitoring & Validation
- Ongoing monitoring of:
– Particulate counts (non-viable)
– Microbiological levels (viable organisms)
– Temperature, humidity, and pressure
- Initial and ongoing qualification (IQ/OQ/PQ) and periodic re-certification by accredited third-party auditors.
Engineering and Operational Challenges
Establishing ISO Class 7 capabilities involves:
- Capital Costs: Construction of cleanrooms can cost $1,000–$1,500+ per square foot (vary based on several factors), not including cleanroom-compatible machinery.
- Tool Design Constraints: Moulds must avoid dead zones, generate minimal flash, and be suited for cleanroom maintenance.
- Workforce Training: Requires SOPs, validated cleaning regimens, and continuous compliance training.
- Environmental Controls: Maintenance of HVAC systems, pressure regimes, and validation data logs is both complex and ongoing.
Given the cost, complexity, and stringent operational requirements, ISO Class 7 cleanroom injection moulding is generally limited to high-tier manufacturers with long-term vision and deep technical infrastructure. It is often seen as a threshold capability for entering regulated medical device markets, particularly those involving Class II/III devices or diagnostic consumables.
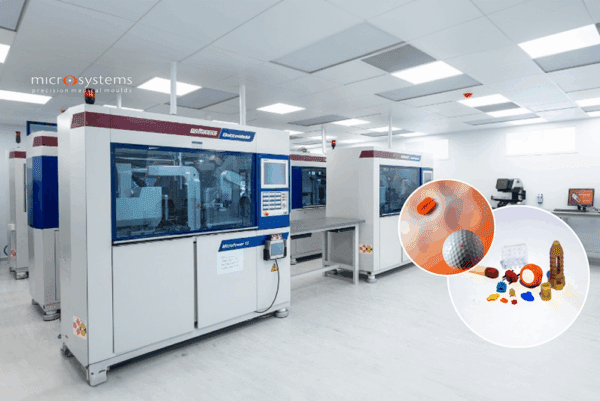
Cleanroom Injection Moulding at Micro Systems
To meet rising demand for high-precision medical components, Micro Systems made a strategic investment in a purpose-built ISO Class 7 cleanroom injection moulding facility. Designed and delivered by Airology Systems, the new environment supports our business expansion by providing increased production and administrative space within a state-of-the-art facility.
Built to ISO 14644-1 standards, the cleanroom features advanced temperature control,
HEPA filtration, specialist extraction, and pressure monitoring systems. Particular focus was given to room layout and integration of a custom extraction solution to support our latest precision moulding and metrology equipment.
The facility also includes 24/7 remote environmental monitoring, ensuring full traceability and control from anywhere in the world. With these new capabilities, Micro Systems is well-positioned to scale production and continue delivering high-quality components for today’s most demanding medical device applications.

stage of manufacture, with particular focus on accommodating new, state-of-the-art equipment through a bespoke extraction system. The completed project not only delivered a fully operational cleanroom and office area but also enabled Micro Systems to scale up production, monitor environmental conditions remotely 24/7, and confidently meet the growing needs of their business.”
Conclusion
Cleanroom injection moulding under ISO Class 7 conditions is more than a process enhancement, it is an operational necessity for companies seeking to lead in the medical device sector. It represents a commitment to patient safety, regulatory compliance, and technical excellence. While the barrier to entry is high, the payoff, access to premium markets, minimised risk, and long-term OEM partnerships, is unmatched.
As medical devices become smaller, smarter, and more integrated, manufacturers who invest in true cleanroom capabilities today are positioning themselves at the forefront of tomorrow’s healthcare technology.
Get more news from Micro Systems here.
Micro Systems
+44(0)1942 290 960
Website
Email