MedTech Focus Pays Off for Kistler at Interplas 2021
Kistler reported three busy and successful days on stand G26 at Interplas, with a steady stream of visitors requesting more information about its recently released products for the MedTech industry.
Kistler is a global market leader for dynamic pressure, force, torque and acceleration measurement technology, with cutting-edge technologies providing the basis for the group’s modular solutions. Customers in industry and scientific research benefit from Kistler’s experience as a development partner, enabling them to optimise their products and processes to secure a sustainable competitive edge.
Kistler showcased its ComoNeo cavity pressure monitoring and maXYmos manufacturing process monitoring systems at the Interplas 2021 exhibition. Kistler has seen increased interest from the MedTech sector since the introduction in late 2020 of the new medical compliant versions of the systems.
ComoNeo 4.1 has several new features needed to comply with the medical market requirements, including LDAP Windows authentication and extra logging to store all device changes. The ComoNeoPREDICT, a DoE model-based system, uses AI and supervised machine learning algorithms based on neural networks to predict the characteristics of injection moulded parts.

As more injection moulders are providing assembly services to their MedTech customers, validating the manufacturing process becomes essential. The Kistler MaXYmos ML, the first process monitoring system to meet the strict FDA and the new European Medical Device Regulations (MDR) for quality assurance in the MedTech sector, simplifies the validation process by delivering 100% confirmation that each step in the manufacturing process has been carried out within the product specification.
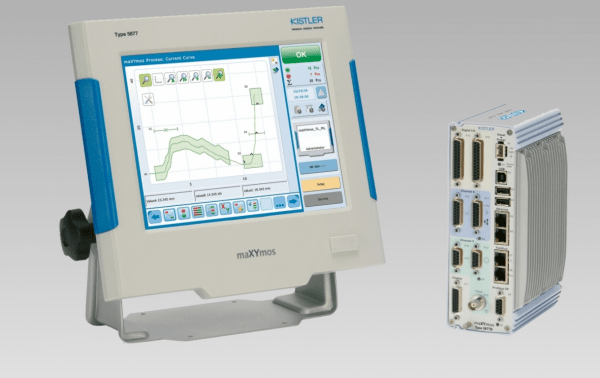
According to Duncan Webster, stand manager and Kistler Applications Engineer, there was much interest in the unique combination of Kistler’s ComoNeo 4.1 cavity pressure monitoring system and MaXYmos ML process monitoring system from the injection moulding community serving the MedTech sector.
The new products ensure conformity with both FDA and European MDR whilst delivering higher productivity, zero rejects and accurate costings.
Click here to find out more about Kistler’s services, products and solutions.
Kistler UK
+44 (0) 1256 741 550
Website