From Prototype to 1 Million Parts: Scaling Medical Device Production with a Single Supplier // Micro Systems Blog
At Micro Systems, we understand that the biggest challenge in medical device manufacturing isn’t creating a single prototype, it’s scaling that design into millions of compliant, reliable parts. Every stage between concept and full production carries technical and regulatory risk, from tooling and material validation to cleanroom requirements and process qualification. In this blog, we explain why working with a single, ISO 13485–certified supplier from the outset reduces risk, streamlines validation, and ensures a faster, smoother route to market.
Medical device innovation often begins with a single prototype. But turning that concept into millions of medically-compliant parts is a complex journey that demands tight quality control, regulatory compliance, and cost efficiency. Partnering with one qualified manufacturing supplier from the outset can streamline the entire process, from early design iterations to high-volume production, while reducing risk and accelerating time-to-market.
Why a single-supplier model reduces risk
Medical devices must meet stringent performance, safety, and regulatory standards. Transitioning from a functioning prototype to high-volume production is not simply a matter of “making more parts.” Manufacturers must carefully address tooling strategy, material validation, cleanroom requirements, automation, and complete regulatory documentation. Errors or inefficiencies during this transition can lead to costly delays, failed audits, or product recalls. Scalability must therefore be embedded into the product design and supplier engagement strategy from the earliest stages. That approach creates multiple points of failure:
- Design interpretation gaps: CAD data or critical tolerances can be lost in translation between design and mould makers.
- Redundant validations: A new production vendor must repeat IQ/OQ/PQ, adding months to launch timelines.
- Fragmented traceability: Regulatory audits become more complex when quality records are distributed across multiple entities.
A single, ISO 13485–certified partner eliminates these risks by maintaining a continuous digital thread, from initial CAD through final device lot release.
Steps to scale Medical Device manufacturing
Scaling medical device production is not achieved in a single step, it is a carefully managed, sequential technical journey. A technically proficient and integrated supplier will navigate you through each critical stage:
- Concept & Design for Manufacturability (DFM): Collaborative engineering at the earliest stage ensures the device is conceived with manufacturing constraints, material selection, and regulatory requirements in mind. DFM analysis identifies tolerances, assembly considerations, and cost drivers before they become issues.
- Rapid prototyping: Advanced additive manufacturing, precision CNC machining, soft tooling or single-cavity mould tooling enable rapid iterations. These prototypes are used not only for form, fit and functional assessment, but also for early biocompatibility checks and usability testing.
- Pilot production & process validation: Limited-volume manufacturing validates the robustness of the chosen processes. Critical process qualification, IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification), confirms that equipment, workflows and controls can consistently meet specification.
- Tooling & automation: High-precision injection moulding tools and other bespoke fixtures are developed, with consideration for long-term durability, maintenance, and reproducibility. Automation and in-line inspection systems are introduced to improve repeatability, reduce operator variability, and prepare for high-volume output.
- Regulatory alignment: Every stage is documented within a quality management system conforming to ISO 13485 and aligned with FDA and MDR expectations. Validation protocols, risk assessments, and device master records are established to support regulatory submissions and audits.
- Full-scale production: Once validated, manufacturing is scaled to hundreds of thousands or millions of units. Real-time statistical process control (SPC), in-line metrology, and automated data capture ensure traceability and consistent quality across production batches.
- Full lifecycle support: Beyond manufacturing, a capable supplier provides continuous lifecycle support including engineering change management, cost optimisation, revalidation where necessary, and end-of-life planning. This ensures the device remains compliant, competitive, and reliable throughout its market presence.
This typical structured, technical pathway mitigates risk, minimises cost over-runs, and ensures that the device is not only manufacturable but compliant, reliable and scalable for sustained market supply.

Key capabilities to look for in a Medical Device supplier
When selecting a supplier to scale from prototype to mass production, prioritise:
- ISO 13485 certification and/or FDA registration
- In-house prototyping technologies (3D printing, CNC machining, injection moulding)
- Pilot production and process validation capabilities
- Full traceability and documentation systems
- Global supply chain and logistics management
- Cleanroom assembly and sterile packaging options
Tips for a successful prototype-to-volume Medical Device manufacturing journey
Making the leap from prototype to high-volume medical device manufacturing requires more than engineering expertise, as it demands strategic planning, regulatory alignment, and strong supplier partnerships. To ensure a smooth and compliant scale-up, consider the following best practices:
- Engage medical device manufacturing experts early to integrate Design for Manufacturability (DFM) and reduce the risk of costly design changes once regulatory submissions have begun.
- Select medically approved materials strategically, prioritising biocompatibility, sterilisation compatibility, regulatory acceptance, and long-term availability to guarantee consistent production and patient safety.
- Validate processes at the pilot stage through IQ, OQ and PQ testing before committing to production tooling, ensuring scalability, compliance, and regulatory audit readiness.
- Maintain transparent, continuous communication with your medical device supplier, particularly around quality management systems (QMS), risk management, and timelines to prevent compliance gaps.
- Leverage advanced digital manufacturing tools, such as CAD-to-production integration, automated traceability systems, and real-time process monitoring, to strengthen quality control and regulatory documentation.
By finding the right turnkey Medical Device supplier and maintaining a strong partnership from day 1, MedTech companies can hence minimise delays, reduce compliance risks, and accelerate time-to-market, transforming a single prototype into millions of safe, reliable, and regulatory-approved medical devices.
How Micro Systems supports scalable Medical Device manufacturing
Micro Systems specialises in taking medical device projects from early-stage prototyping all the way through to high volume production. With decades of experience in ultra-precision mould design and manufacture, micro moulding and cleanroom manufacturing, Micro Systems provides:
- End-to-end support from design and prototyping to full-scale production
- In-house tooling expertise for ultra-precise components
- ISO 13485 and 9001-certified facilities ensuring compliance at every stage
- Global manufacturing capabilities to scale production rapidly
- In-house metrology and quality assurance capabilities that guarantee consistency across millions of parts
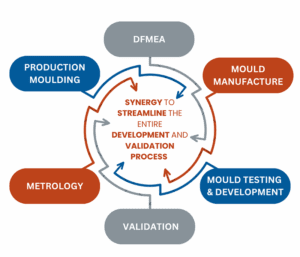
By partnering with Micro Systems, MedTech companies gain a trusted single-source supplier that reduces risk, accelerates regulatory approval, and ensures smooth scaling from prototype to over 1 million parts.
Micro Systems will be exhibiting at Medical Technology Ireland on 24–25 September at Galway Racecourse! Join us at Stand 322 to explore how our precision micro moulding expertise can accelerate your medical device innovation. Whether you’re looking to discuss new projects or explore advanced manufacturing capabilities, we’d love to chat. To book a meeting in advance, email us at info@microsystems.uk.com.
Get more news from Micro Systems here.
Micro Systems
+44(0)1942 290 960
Website
Email