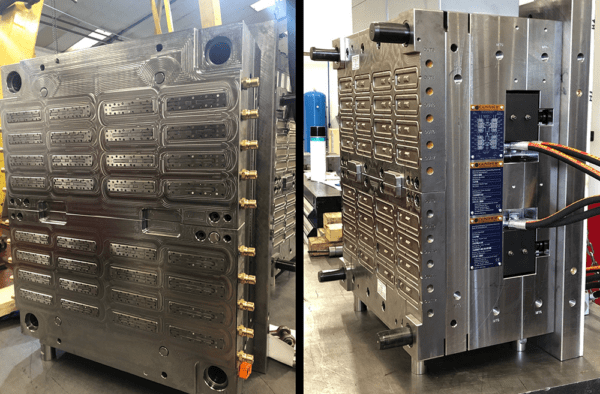
Bringing the Lateral Flow Device to Market – GÜNTHER UK Case Study
It seems a lifetime ago now – but in 2020 the call went out for a lateral flow device to be used widely as a detection mechanism for Covid-19. With their partners at Guenther Heisskanaltechnik in Germany, GÜNTHER UK was thrilled to be a part of the solution on one project in particular.
The test is a hand-held device with a plastic top and bottom casing enclosing a composite strip onto which various chemicals are impregnated. A blood sample mixed with a reagent is introduced through one opening and flows along the strip, causing a line to appear at a specific position in a viewing window if it is a positive result approximately 20 minutes later.
This is a press release that went out at the time:
In March 2020, Amies Innovation of Chesterfield, Derbyshire were asked by an existing customer to look at the Top and Bottom Cover parts for a Lateral Flow Device (LFD), as they were already involved in manufacturing similar components they were a natural partner for this project.
Established in 1929, Amies is a technical moulder with many years of experience in the development and manufacture of medical devices and other tightly toleranced plastic parts.
The medical moulding division holds ISO 9001:2015 and ISO 13485:2016 quality management standards for medical components and assembly as well as having the modern quality testing and measuring equipment that are critical for this kind of application.
Production requirements in the pandemic meant that initially, one 8-impression tool was required for the top cover and another one for the bottom, followed up by 2 x 32 cavity moulds. Of course, the pandemic required that mouldings were able to be supplied in the shortest possible time and the challenge for Amies was then to also identify the technically capable toolmaker and hot runner supplier with whom to work to achieve these goals.
Because of the importance of these parts to the fight against COVID-19 Amies, the toolmaker RDMS from Oldham and GÜNTHER hot runner Systems worked together to ensure that the 8 impression tools, including full valve gate hot runner, were delivered in just 5 weeks and even the completed 32 cavity moulds in an impressive 10 weeks.
Simon Stewart-Smith, Technical Director of Amies says: “Having worked with RDMS closely over the years on a number of technically demanding projects, there was no doubt they would be the correct partner for this project, with the ability to produce high-quality tooling in a very challenging lead-time. Similarly, having worked with GÜNTHER on other high spec. medical and automotive projects in the past, I knew their Valve gate technology was the right one for our project. Knowing the importance of the project in the context of Covid 19, although already very busy, both companies pulled out all the stops to achieve very difficult lead-times for which they must be commended.
“In the background to all of this, Amies also needed an additional new press to support the manufacturing volumes. Long-term trusted machinery partner Arburg, who supply all Amies injection moulding machines, were able to provide exactly what was needed to meet the project timelines.
“To complete the picture we also needed some high-quality cooled conveyors with integrated box handling automation which UPM Conveyors again stepped up and provided in the required timeline to a high-quality standard”.
Chosen toolmaker Manchester-based RDMS, had developed similar tooling in the past where push fits were also critical and were therefore able to take a clinical approach to obtain first-time parts. They are able to take CAD data for the new part along with similar existing parts already produced by Amies and verify CAD vs part with high accuracy, guaranteeing they would match each other to give the required push fit.
To keep competitive, RDMS use an unmanned running system, which combined with their choice of machines and automation, enables them to machine and spark erode to a very high accuracy. The use of their 3 CMM machines verifies the tooling surfaces for quality and accuracy which helped with the fast lead times required for these tools.
Neil Richardson, director of RDMS, says “Our customer Amies wanted to hit the ground running for obvious reasons and so choosing a hot runner company that would respond quickly and who offer committed technical support to feeding these parts was important and GÜNTHER was chosen. Designing and manufacturing the 8 impression tools in 5 weeks followed by 2 x 32 cavity moulds in just 10 weeks was very demanding and we were very proud to be part of this successful project”.
Valve gate systems from leading German hot runner supplier GÜNTHER were chosen for this project based partly on successful medical projects undertaken between GÜNTHER and Amies in the past, and because of the company’s extensive experience in the supply of systems for medical device manufacture worldwide. Requirements of the hot runner system were particularly for clean gate vestiges, cavity balance across all parts and reliability.
All GÜNTHER nozzles feature patented bi-metallic titanium shafts which ensure a very homogenous temperature profile across the whole length of the nozzle meaning the material is delivered to the cavity in its best possible condition. Standard nozzle type 6NMT were used with the heads machined to achieve the required small pitch centres. GÜNTHER valve gate systems also feature unique PM insert technology, essentially a replaceable needle guide and gate insert.
Tolerances on these are extremely tight which, in combination with the homogenous temperature profile, gives the moulder the required high-quality gates. Another big benefit of the PM inserts is that costly reworking of gates over time is avoided they can be easily exchanged without stripping the whole hot runner. Manifolds are fully balanced and activation is via a single lifting plate to ensure all valve pins open and close at the same time.
Via small access holes in the back valve pins with the GÜNTHER system can be exchanged without even taking the back plate off. Deliveries of these systems were brought forward because of the importance of tackling Covid and the 8 drop systems took just 4 weeks and even the 2 x 32 cavity hot runners were delivered in just 5 weeks. The tight delivery schedules meant that a hot half option was not considered and the systems were all installed, wired and tested by the engineer of GÜNTHER UK at RDMS.
Reiner Heendeniya, Sales Director of GÜNTHER UK, says “We were all trying to help in the fight against COVID-19 and there is no denying that the technical solution and delivery, including design, was a challenge but it was one we rose to and it was a real achievement to be moulding full production parts in high volumes in such a short space of time.”
Read more from GÜNTHER here.
GÜNTHER UK Limited
01474 879774
Website
Email